pH Indicators in Nature
KITCHEN SCIENCE: October 24, 2024
CONCEPTS
Ph and color change in foods
Acids and Bases
TOOLS & SUPPLIES
Butterfly pea powder
One lemon
Baking soda
Clear cup
Using butterfly pea powder to see pH
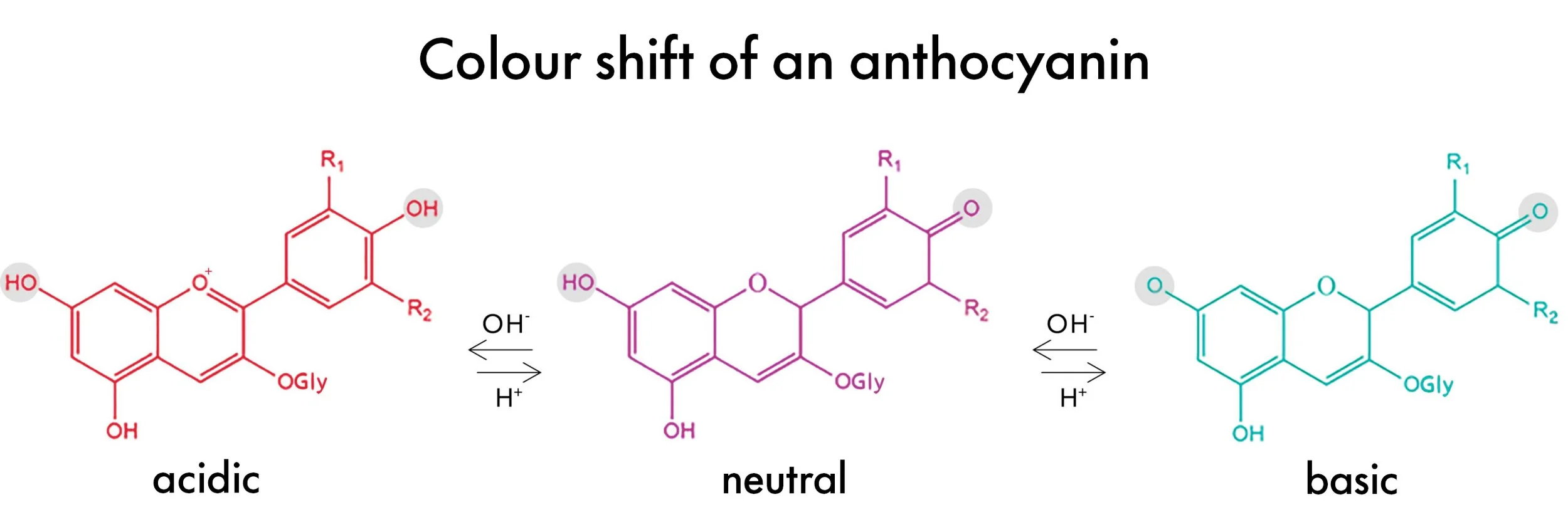
Butterfly pea flowers contain a bright blue pigment called anthocyanin, which acts as a pH indicator. The color changes when an acidic or basic solution is added.
METHOD
1. Prepare the “tea”:
a. Steep a few butterfly pea powder in hot water for a few minutes until the liquid turns a deep blue color.
2. Observe the initial color:
a. Note the initial blue color of the solution.
3. Add acid:
a. Slowly add a few drops of lemon juice to the solution and observe the color change from blue to purple or pink.
4. Add base (optional):
a. To demonstrate the reversible nature, add a small amount of baking soda to the acidic solution and watch the color shift back towards blue.
Hydrangea flowers are a common plant to see in yards all around the world! Some varieties of Hydrangea change based on the pH of the soil they are growing in.
The color change in Hydrangea flowers is actually due to the presence or absence of aluminum compounds. The soil pH indirectly changes flower color by affecting the availability of aluminum in the soil.
When the soil pH is lower (or more acidic), plants have an easier time absorbing nutrients, including aluminum. For Hydrangeas, this results in blue flowers!